Radiolysis and UV photolysis generate radicals
Radicals can be generated by many different types of particles. In fact, some of the most promising work in the abiotic synthesis of RNA precursors using UV light relies on the production of solvated electrons, which readily generate radical species. UV light can also generate an array of oxidizing radicals. The use of light near the visible spectrum to break apart molecules to drive chemical reactions is known as photolysis, and the use of particles associated with radioactive decay events to drive such reactions is known as radiolysis.
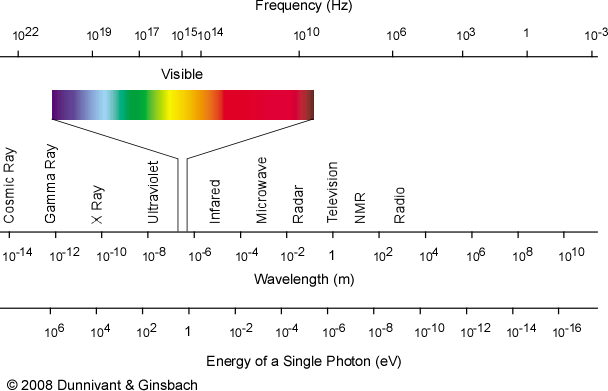
Since the formation of radicals involves the breaking of chemical bonds, this implies that the input energy per particle ought to exceed the typical bond energy for a chemical compound. Many common bond energies are on the order of ~1-10 eV, which corresponds to photons ranging from the near infrared, through the visible, and into the near ultraviolet portions of the spectrum:
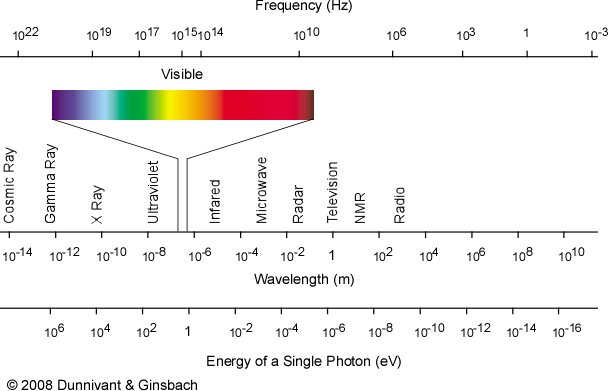
Beyond this basic implication, though, the story gets more complex. Energy from this part of the spectrum can be absorbed, attenuated and quickly redistributed by atoms and molecules in many different ways at standard temperatures and pressures. Incident energy from this part of the spectrum is therefore more likely to produce chemical or physical outcomes (like heating or excitation of vibrational modes) that do not readily yield radicals. This is a good thing for the persistence of life, because if bonds could be broken and radicals formed so readily, it is difficult to imagine biomolecules like DNA, RNA or proteins lasting very long!
But this is just the beginning of the story, not the end of it, for one main reason: the conditions under which life can persist may not not be so effective at originating life from scratch. Radical production greatly increases in settings where more powerful types of energy are more abundant (such as during intense solar storms, in proximity to radioactive minerals, and in the space environment), or where the ambient pressure is very low (such as in the upper atmosphere). These settings may be less familiar to us, and they may occur under less common conditions, but they are nevertheless found everywhere throughout the solar system and are promising conditions under which to study life’s origins. Radical-generating sources can include charged particles (alphas, betas, protons, atomic nuclei, etc.) as well as neutral species such as photons that can range from the UV up to gamma rays.
For these reasons, REPO focuses on this powerful, less-geochemically-explored part of the spectrum (UV-equivalent energies and up) because it is the regime of ready bond dissociation and radical recombination.