Radicals generate innate cycles
The basic structure of radical production and consumption shows that cycles are an inherent feature of radical-driven systems. Consider the relatively simple system of chlorine radical production and interaction with a simple molecule such as methane:
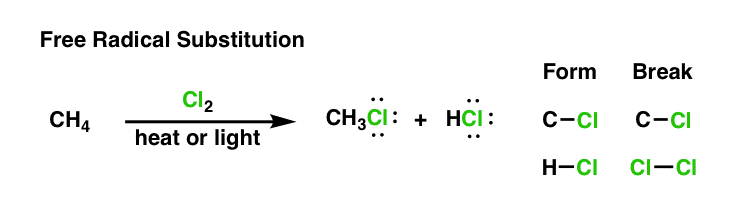
At face value, this is a straightforward process where the Cl2 molecules are broken down into two Cl atoms, which cleaves an H atom off of CH4, and results in the production of CH3Cl and HCl. The equation representation of this process is linear and a fairly accurate approximation of the outcome.
But look what happens when the the intermediate steps that lead to this outcome are explicitly depicted:
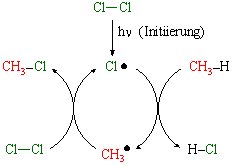
The inputs and outputs are the same, but the connection between the inputs and outputs are facilitated by 2 half cycles and one complete cycle. Variations in this simple topology are found in nearly every system where radicals are produced. The pattern is subtle but very important: it means that radical systems are inherently cyclic in nature.
This pattern arises from the basic lifetime of radical processes, which is broken into three distinct phases. In the initiation phase, a molecule is split into two fragments, each with an unpaired outer valence electron. In the propagation phase, each of these radicals interacts with other compounds around it, each time generating a new radical. In the termination phase, two radicals combine to create a non-radical product. It is the propagation step that can lead to cycles, because the only way to end a radical chain is through combination with another radical. Until a recombination occurs, an intermediate radical reaction can only lead to the production of another radical, and each of these intermediates can possibly lead to different, final products in the termination step.
Radicals formed as intermediates in this way can form emergent cycles of many different sizes and topologies, particularly as the number and concentration of different products diversify in a radiolytic system. We hypothesize that this property can eventually lead to autocatalytic cycles (cycles in which one compound is doubled with every turn). Autocatalytic cycles are important, possibly foundational, chemical relationships that are thought to have been involved in life’s origins.
The basics of this discussion were adapted from a more detailed treatment of radicals in a paper by Studer and Curran (2016).