Extreme Electron Potentials
Radicals can have extrema of electron reduction potentials, both positive and negative. These extrema can greatly exceed values associated with compounds thought to be highly reducing (such as sugars) or highly oxidizing (such as oxygen gas, O2). Let’s put this into perspective. The following is a plot of electron reduction potentials associated with a collection of common metabolic substrates and intermediates from Karp (2009):
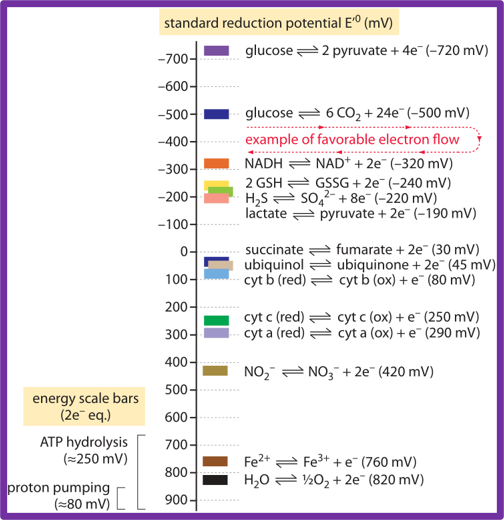
As you can see, excellent electron donors such as sugars are found around -600 mV, while efficient electron acceptors such as oxygen are found around +800 mV.
Now, let’s see where some common radical species are found relative to this scale, as listed in Armstrong et al., IUPAC Technical Report PAC-REP-14-05-02. The plot above is again outlined in a solid purple box, but expanded to include common radical species and the solvated electron:

The potentials associated with very common radicals such as H (-2300 mV) or OH (+1900 mV) exceed those used in metabolism by many multiples. This difference in energy levels is extremely significant if we want to evaluate the possibility that radical-driven systems can overlap with conventional redox-driven reactions. For systems in which energy is distributed probabilistically in proportions related to e^[-deltaEo/kT], the chemical pathways that are driven by radicals are essentially inaccessible to redox-powered reactions (perhaps on the order of 1^-22). In other words, they do not probabilistically overlap in any way. The only way to probe radical chemosynthetic potential is through experimentation.
What this also indicates is that systems driven by powerful radicals can lead to systems with no redox potential ‘dead-ends’. Almost any bond created under plausible biotic or prebiotic intermediate compounds can probably be reacted with radical compounds; some more quickly or slowly than others, but over time all compounds produced at one point in time can and probably will be interconverted to another compound at a later time.
As long as it is probable that no compound or bond in a radical-powered system will be a ‘dead-end’, there is a high probability that chemical reaction cycles of formation, degradation and interconversion will eventually be formed for all compounds. It is therefore more likely that such a system is open-ended on long time scales.